Meet The Rarest Element On Earth – Astatine
 Short Bytes: Astatine, which is rarest element on Earth, has a half life of just 8.1 hours. Astatine is so rare that there’s less than 30 grams of it in the Earth’s crust. Let’s tell you more about it.
Short Bytes: Astatine, which is rarest element on Earth, has a half life of just 8.1 hours. Astatine is so rare that there’s less than 30 grams of it in the Earth’s crust. Let’s tell you more about it.
For instance, Astatine is so rare that there’s less than 30 grams of it in the Earth’s crust. In fact, what more contributes to its rarity is that, till date, scientists could produce only 0.05 micrograms of it. This is owing to the fact that to use it, they need to produce it from the scratch.
Also read: Earth’s Magnetic Field May Not Flip-Matter Of Relief Or Concern
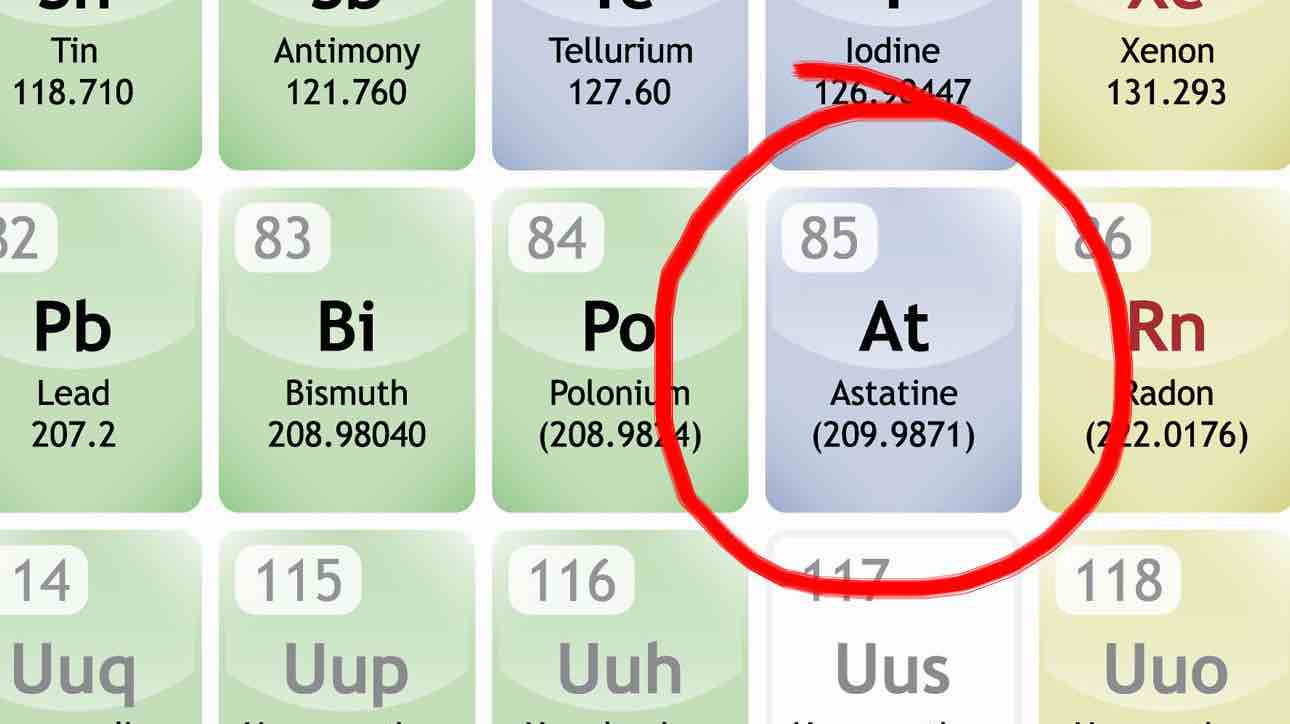
Astatine, which has derived it’s name from the Greek word for unstable- “astatos” is a naturally occurring semi-metal that results from the decay of uranium and thorium. In its most stable form – astatine-210 – it’s got a half-life of just 8.1 hours. So, by the time one wakes up from sleep, it would have gone down by half! Depending on how it decays, it’ll either turn into the isotopes bismuth-206 or polonium-210.
Have a look at the following chart:
Also, as it would be vaporized by the heat of its own radioactivity, scientists assume it would take on a dark or metallic appearance if one could see it. Also, it is the rarest naturally occurring element that isn’t a transuranic element! Transuranic elements are the chemical elements of atomic number greater than that of uranium (at. no. 92) in the periodic table.
Of the known transuranium elements (23 as of 1999), only two-neptunium and plutonium-exist at all in nature; the others have been synthesized through nuclear reactions involving bombarding the atoms of one element with neutrons or fast-moving charged particles.
On this, From Quarks to Quasars says:
“Because the transuranic elements have half-lives much shorter than the age of our planet. As a result, if any of these elements were ever present on Earth, they have long since gone, decaying into other things. As a result, if any of these elements were ever present on Earth, they have long since gone, decaying into other things.”
Tell us your opinions in the comments below.
[adinserter block=”12″][adinserter block=”13″]